Atmospheric Corrosion

What is atmospheric corrosion?
Atmospheric corrosion—perhaps the corrosion phenomenon we experience more frequently in our daily lives—refers to the “situation of exposure of a component.” Although uniform corrosion (e.g., rust) is to the most common form of atmospheric corrosion, atmospheric corrosion does not imply a particular type of corrosion.
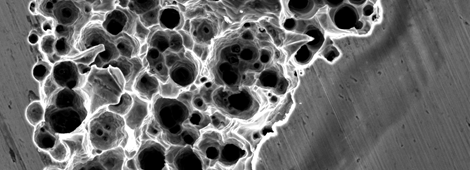
Indeed, atmospheric corrosion may lead to localised corrosion, stress corrosion cracking (SCC), and corrosion fatigue. The figure in this page shows an example of atmospheric SCC that led to the collapse of a swimming pool roof in Uster, Switzerland, May 1985. SCC of stainless steel rods in tension was promoted by the exposure to a chloride-rich atmosphere despite the low temperatures. As a result of the failure, twelve people lost their lives.
Factors affecting atmospheric corrosion
Atmospheric corrosion occurs due to the presence of a thin film electrolyte. Dissolved oxygen, the primary cathodic reaction in most atmospheric corrosion cases, is more readily transported through a thin film than in bulk solution.
Atmospheric corrosion is typically described in terms associated with climatology, such as rainfall, relative humidity, pollutants, winds, aerosol transport, temperature, etc. In short, the rate of atmospheric corrosion depends upon the length of time moisture is in contact with the surface, the degree of pollution of the atmosphere, temperature, and the chemical composition of the substrate, e.g., carbon or stainless steel.
Unprotected surfaces that are in contact with moisture and exposed to the environment for extended periods are prone to atmospheric corrosion. The most common approach to mitigate uniform atmospheric corrosion is by the use of external barrier coatings to prevent the direct exposure of the base metal to the environment. Nonetheless, coatings also degrade due to exposure to the atmosphere, where Ultra Violet radiation, heat, and pollutants play decisive roles.
Atmospheric corrosion research at Curtin
At the Curtin Corrosion Centre, we study coatings and materials degradation caused by atmospheric exposure using a combination of fundamental electrochemical methods and simulated exposure cycles in our salt spray and environmental chambers.
In our studies, we can simulate specific outdoor atmospheric conditions, including extreme temperature cycles, UV exposure, marine atmospheres, intermediate immersion, and more.
Accelerated tests are used to study the rates of degradation of various coatings in specific environments. Life prediction models based on a combination of electrochemical methods and changes in physical properties after long-term exposures are also being developed here at Curtin Corrosion Centre.