Localised Corrosion
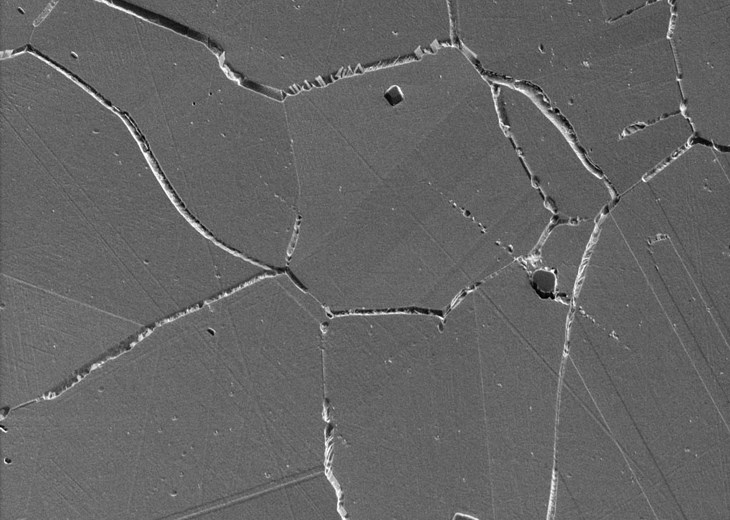
Passivity
As stated by Prof. Digby Macdonald, “humankind has been able to develop a metals-based civilization primarily because the reactive metals (Fe, Ni, Cr, Al, Ti, Zr...) exhibit extraordinary kinetic stabilities in oxidizing environments.” The concept of passivity is more than 200 years old, initially described as a curious phenomenon in which a reactive metal would behave like a noble metal in conditions where active corrosion would be expected.
We now know that passivity is the loss of electrochemical reactivity due to the formation thin, i.e., a few nm thick, robust oxide layers. Most engineering alloy systems such as stainless steels, nickel-, titanium-, and aluminium alloys exhibit passive behaviour under certain conditions. A passive state does not imply the absence of corrosion but a drastic reduction in corrosion rates, which often falls between 0.1 to 1 μm/year. For example, at 0.1 μm/year it would take more than 16,000 years to corrode a typical coin uniformly.
Breakdown
Passive metals and alloys that are typically corrosion resistant are often susceptible to localised corrosion as a result of the local breakdown of the otherwise protective passive film.
Pitting and crevice corrosion are localised forms of attack that can propagate at fast rates and can be difficult to detect. During localised corrosion, most of the surface remains unaffected, while pits could be growing under “apparently inoffensive corrosion products.” Similarly, corrosion occurring at crevices (i.e., mating surfaces at least one being metallic) or under deposits is shielded from direct view. Localised corrosion can frequently continue until the full wall thickness penetration of a component occurs, leading to leaks and containment losses.
Metallurgical factors
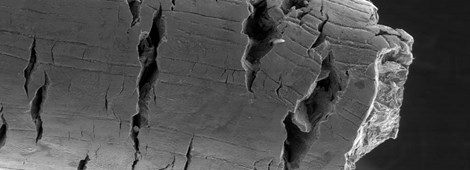
Localised corrosion is highly influenced not only by the composition of an alloy but also by heat treatments and manufacturing parameters. In this regard, the precipitation of, e.g., chromium carbides and nitrides, σ-phase and other so-called deleterious phases drastically reduce localised corrosion resistance, leading to other forms of localised corrosion such as intergranular attack. The photo in this page is an example of intergranular corrosion of an age-hardened nickel alloy that was “sensitised” by the precipitation of grain boundary phases rich in Cr and Mo.
Stress Corrosion Cracking
It has been shown that in, e.g., oil and gas environments, pitting corrosion of certain corrosion-resistant alloys can serve as a precursor to stress corrosion cracking (SCC) and other forms of environmentally assisted cracking. It is known that when the pit propagation rate meets a certain critical value, fast crack propagation can initiate from the bottom of pits. However, the mechanisms that lead to the initiation of SCC are yet to be resolved.
Localised corrosion research at Curtin
Our team at the Curtin Corrosion Centre has gained international recognition in the field of localised corrosion of stainless steels, nickel- and aluminium alloys. Our research covers both fundamental aspects of localised corrosion phenomena as well as the development of practical engineering guidance for materials selection and corrosion management.
As with mechanically assisted corrosion, we focus on linking the performance of an alloy system with microstructure and manufacturing history. We provide expert knowledge on mitigation strategies and risk identification. The Curtin Corrosion Centre is equipped with state-of-the-art facilities to study localised corrosion phenomena from the nano- to the macro-scale.