CO2 Corrosion Inhibitor Performance at Deposit-Covered Carbon Steel and Their Adsorption on Different Deposits.
Overview
Carbon steel pipelines commonly used in the oil and gas industry are known to be susceptible to under-deposit corrosion (UDC). However, the efficiency of inhibitors and their adsorption on different deposits has not largely been addressed. In this study, it was evaluated the inhibitory performance and adsorption of two inhibitors on deposited carbon steel surfaces with aluminium oxide, calcium carbonate and silica sand deposits. It was found that the pyrimidine derivative compound provided the highest corrosion protection on both deposited and non-deposited steels. Also, it was suggested that the adsorption of the inhibitor tended to be related to the inhibitor type and not notably to the physical properties of the deposits.
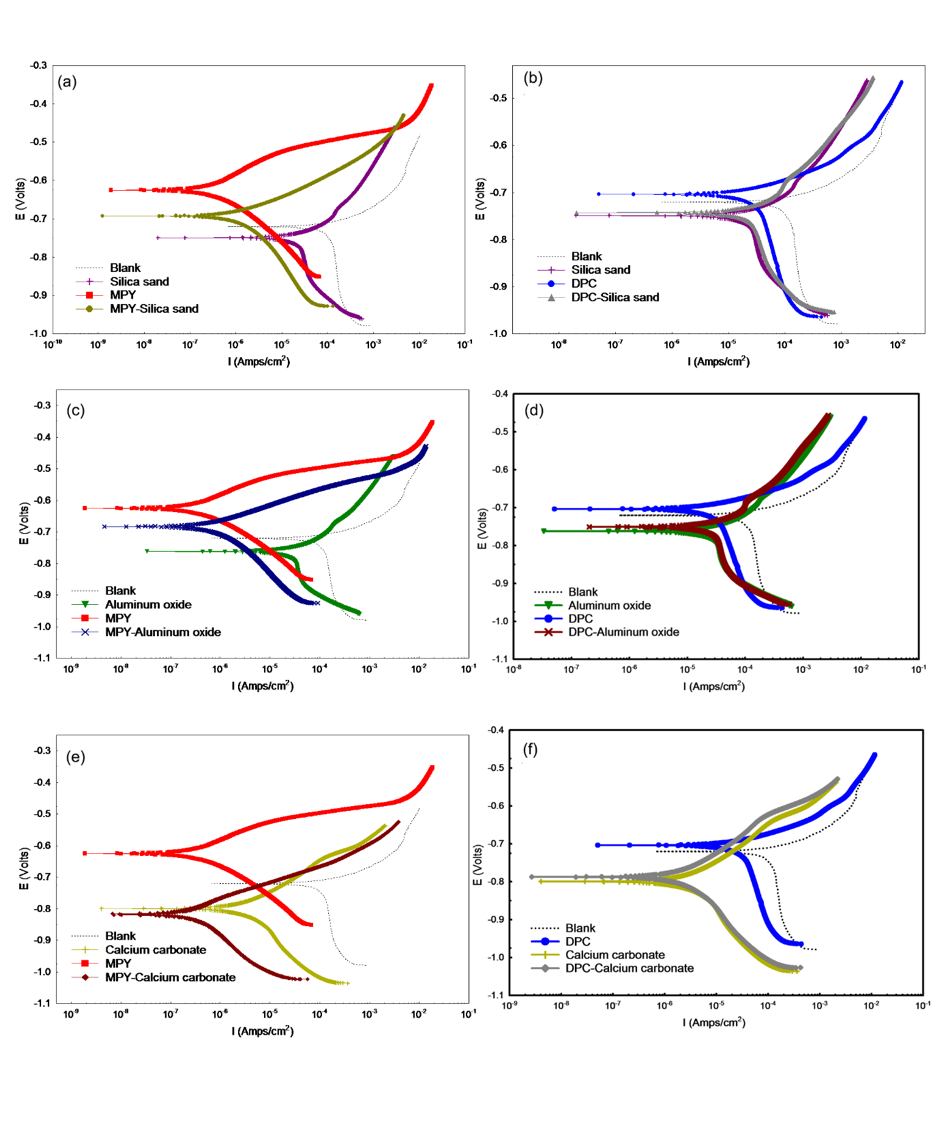
Figure 1; Potentiodynamic polarization curves recorded at bare and deposit-covered-steel surfaces in uninhibited and inhibited solutions (100 mg/L) at 30°C after 24 h immersion period under CO2 environment. Combinations of (a) silica sand and MPY, (b) silica sand and DPC, (c) aluminium oxide and MPY, (d) aluminium oxide and DPC, (e) calcium carbonate and MPY, and (f) calcium carbonate and DPC. In each figure, a blank, the potentiodynamic curve recorded for bare steel exposed in uninhibited test solution has been included for a comparative purpose.
Objectives
This research aims to gain insight into the understanding of inhibitors performance on carbon steel surfaces covered with deposits of different properties, such as mineral type, surface charge, porosity, and particle size.
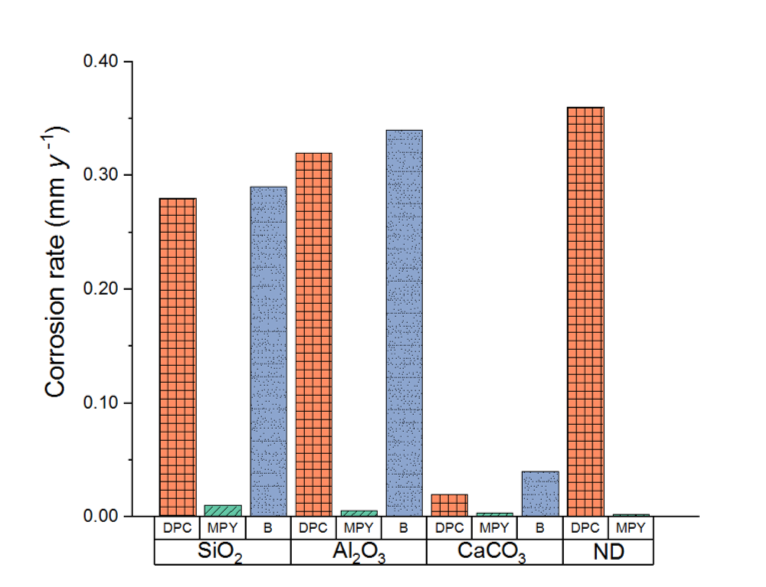
Figure 2; Corrosion rates calculated from potentiodynamic measurements after 24 h of immersion period. B: deposit-covered steel in uninhibited solution. ND: no deposits.
Why is it important?
This research is closely related to industrial applications. It is commonly believed that in optimal conditions an appropriate amount of inhibitor could be enough to provide corrosion protection. However, the presence of deposits can affect the efficiency of a corrosion inhibitor in different ways, for example, by reducing its availability at the steel surface.
Get the paper!
Citation
E.M. Suarez, L.L. Machuca, B. Kinsella, K. Lepková, Corrosion 75, 9 (2019): p. 1118–1127.