Corrosion experiment - Rusting nails

Corrosion of metals is an electrochemical process (Redox reaction) and fundamentally it requires 4 components:
Cathodic reaction (Reduction reaction, positive polarization): A reaction that gains electrons. As a result, the oxidation number decreases. For instance, hydrogen and oxygen reduction reactions are common in acidic and aerated solutions, respectively. The site where the cathodic reaction occurs is called the cathode.
Proton reduction reaction
$$2{H^ + } + 2{e^ - } \leftrightarrow {H_2}(g) \tag{1} \label{eq:1}$$
Oxygen reduction reaction
$$ {O_2}(sol.) + 2{H_2}O + 4{e^ - } \leftrightarrow 4O{H^ - } \tag{4} \label{eq:4}$$
Anodic reaction (Oxidation reaction, negative polarization): A reaction that loses electrons. As a result, the oxidation number increases. Generally, this is the metal dissolution reaction. A metal in metallic form, which has an oxidation number of zero, loses electrons and becomes ions. Metal ions can dissolve away or further react with the surroundings, such as with oxygen to form oxides scales. The site where the anodic reaction occurs is called the anode.
Iron oxidation
$${Fe}\rightarrow{Fe^ {2+} } + 2{e^ - } \tag{5} \label{eq:5}$$
Zinc oxidation
$${Zn}\rightarrow{Zn^ {2+} } + 2{e^ - } \tag{6} \label{eq:6}$$
Electrolyte: a conductive solution to carry ions. Seawater is an effective electrolyte for corrosion due to high salt concentration. Coastal regions are thus more susceptible to corrosion than areas further in land due to deposition of salt aerosols.
Electrical conductor: an electrically conductive medium to transport electrons. For instance, an electrical wire connecting two metal pieces, direct contact between dissimilar metals, or the metal itself.
Different metals have different tendencies to corrode, thus some would corrosion more readily than others (i.e., we say they would be more anodic). In fact, local anodes and cathodes develop even on the same metal surface as they are heterogeneous.
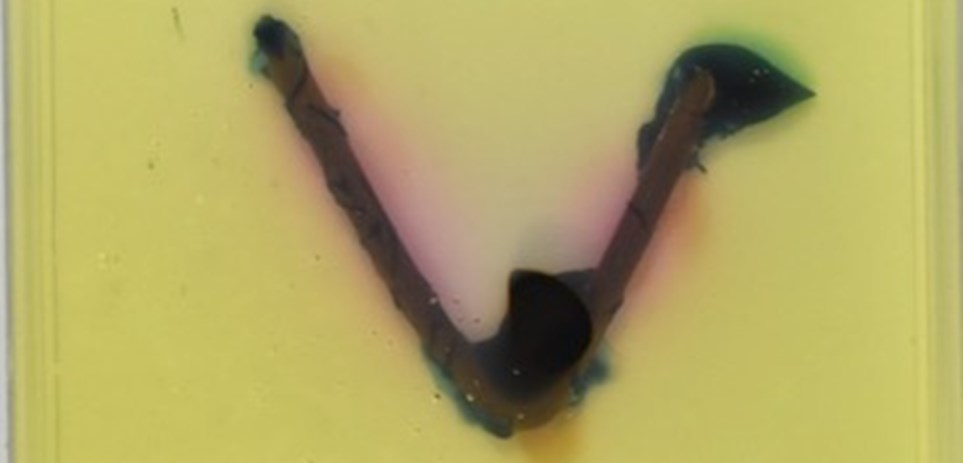
The dissimilar metal corrosion and the effect of heterogeneities in metals on corrosion are demonstrated using steel nails in an agar gel with a pH indicator and ferrous ion indicator. In these examples, the steel nail corrosion was accelerated when electrically connected to copper and was mitigated when connected to zinc. The time-lapse videos show colour changes as reactions proceeded.
Nail - Zinc

Nail - Copper
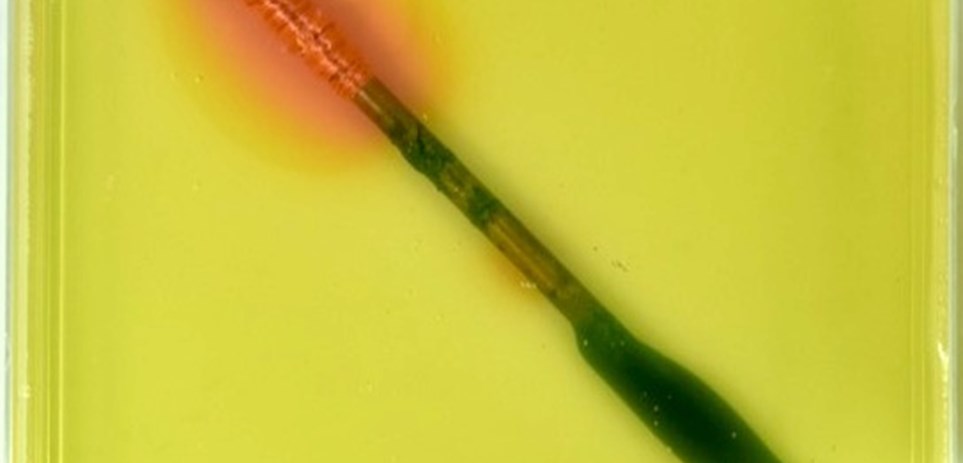
When it is simply just the steel nails in agar, corrosion still occurred. This is because of metal heterogeneities and cold work (bending).
Nail - straight
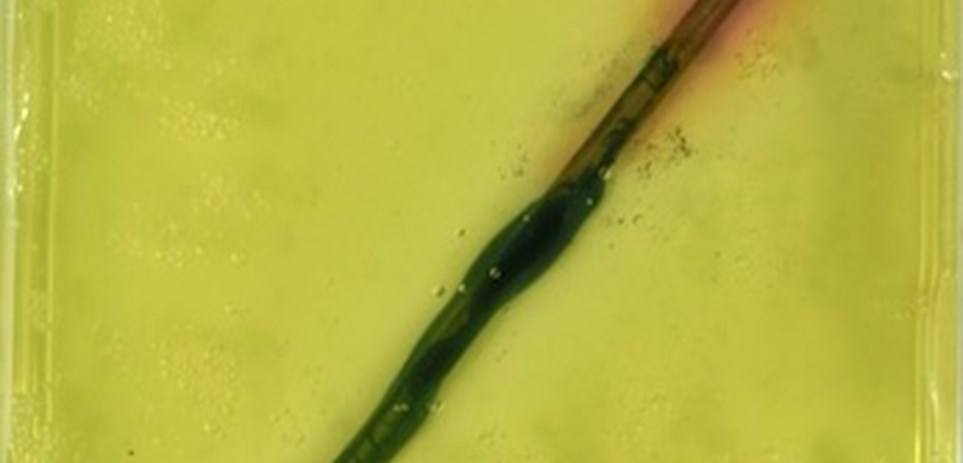
Nail - Bent
We developed a supplementary note to further explain science behind this experiment, some troubleshooting, and additional resources.


