Acid Gas Corrosion
Sweet and Sour
When dissolved in aqueous solutions, acid gases such as CO2 or H2S can be highly corrosive and have led to multiples failures and leakages in the oil and gas industry. These gases are regarded as the primary sources of the internal corrosion of production tubing, transportation pipelines, and sometimes subsequent process equipment. While environments containing predominantly CO2 are regarded as sweet, those that have a sufficient amount of H2S to cause cracking of a material by the mechanisms addressed in ISO 15156 are termed sour.
The severity of corrosion by acid gases depends on various parameters such as type of materials, temperature, the partial pressure, liquid velocity (shear stress), the chemical composition of the liquid phase, etc. Depending on the metal or alloy composition and microstructure, acid gas corrosion can manifest as uniform and localised corrosion. H2S can also lead to environmentally assisted cracking. The different forms of corrosion occurring due to acid gases include, for example, pitting corrosion, mesa-attack, sulfide stress cracking (SSC), and more.
Protective films and localised corrosion
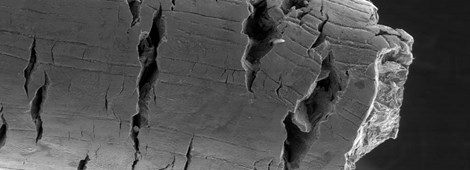
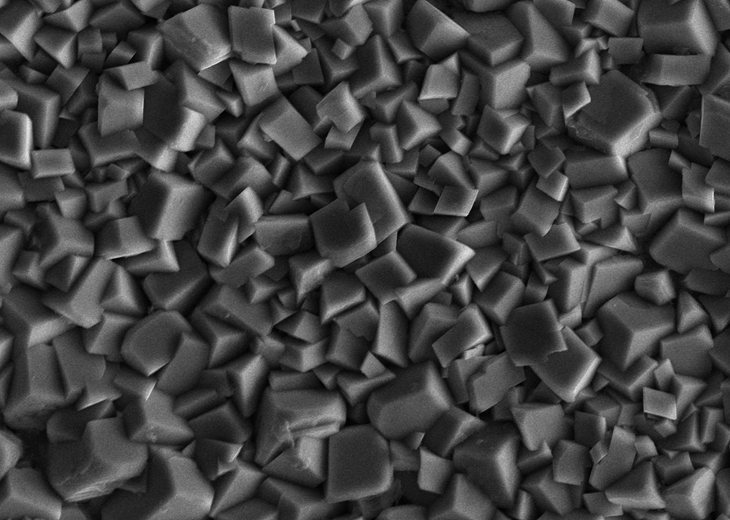
In acid gas corrosion, corrosion products play a critical role. Corrosion films and scales could determine (i) if corrosion is likely to stabilise or accelerate and (ii) the likelihood of transition to pitting or cracking. Iron carbonate films, a primary corrosion product formed in CO2-dominating environments, tend to reduce the uniform corrosion rate of carbon steel by acting as a diffusion barrier. However, localised corrosion can occur when the FeCO3 film is damaged locally or when the water chemistry promotes the formation of a porous layer, as opposed to the preferred compact film.
The presence of H2S leads to complex corrosion reactions. Operating conditions and duration determine the nature of iron sulfides (FexSy) that precipitate on the surface of carbon and low alloy steels. Various stoichiometric ratios between Fe and S may be promoted leading to differences in characteristics and, consequently, corrosion of the underlying metals.
The presence of H2S can, likewise, lead to environmentally assisted cracking in the form of SSC, hydrogen-induced cracking (HIC), stress-oriented hydrogen-induced cracking (SOHIC), and galvanically-induced hydrogen stress cracking (GIHSC). Elongated pits and trenches have been reported in certain types of low alloy steels when exposed to H2S environments. It is uncertain how trenches form and whether they can promote SSC as proposed by some authors.
Acid gas corrosion research at Curtin
Curtin Corrosion Centre houses state of the art research facilities that can simulate complex operating conditions; e.g., from ambient to high-pressure and high-temperature (HPHT) service, from stagnant to turbulence flow regimes, from single phase to two-phase systems (oil/water or gas/water), and from sweet (CO2) to highly sour (H2S) systems. The corrosion behaviour and corrosion prevention of metals exposed to environments containing acid gases are often studied in environments containing CO2, H2S—more often gas mixtures—and temperatures mimicking field conditions.
Our researchers and students are highly trained at conducting complex corrosion experiments and analysing data from electrochemical methods to environmental cracking tests, as well as advanced surface and materials characterisations.
We carry out experiments to industry standards but also tailor experimental designs to meet specific field exposure requirements. Our expertise ranges from fundamental corrosion mechanism studies, failure investigations, to recommendations on corrosion prevention and management.
Effects of Mercury
In collaboration with researchers at the WA School of Mines: Minerals, Energy and Chemical Engineering (WASM:MECE), the Curtin Corrosion Centre has recently developed unique expertise and world-first capabilities to investigate the influence of elemental mercury vapour (Hg0) on the mechanisms, kinetics, and protectiveness of scales formed on carbon and low alloy steels in CO2- and H2S-containing environments.